Source: University of Wisconsin-Madison
Context
Antimicrobial stewardship is a public health concern that calls for the judicious use of antimicrobials in veterinary medicine. The FDA has prohibited certain extralabel uses of cephalosporin and fluoroquinolones drugs in the veterinary field to preserve theseantimicrobials for use in human medicine1. Nowadays, the dairy industry is highly scrutinized due to usage of antimicrobial drugs in dairy cows for preventive or therapeutic purposes because consumers associate these practices with increasing antimicrobial resistance (AMR)2.
What is the difference between antibiotic and antimicrobial?
An antibiotic is a chemical substance, such us penicillin or streptomycin, produced by a microorganism (e.g., fungi) that at a low concentration inhibits or kills other microorganisms. An antimicrobial is any substance of natural, semisynthetic, or synthetic origin that kills or inhibits the growth of microorganisms but causes little or no damage to the host. Thus, an antibiotic fall under the antimicrobial category.
What is mastitis?
Mastitis is the most frequently diagnosed and treated disease in dairy cows. Mastitis is the inflammation of the mammary gland commonly caused by bacteria and is classified as subclinical or clinical based on the absence or presence of clinical signs (abnormal milk, inflamed udder sometimes accompanied by fever), respectively. Overall, pathogens found in the environment surrounding cows are the most common cause of mastitis—one of them is Klebsiella3.

Figure 1. Klebsiella growth on a culture plate that allows differentiang lactose positive from lactose negative fermenting Gram-negative bacteria.
Klebsiella mastitis
Klebsiella are Gram-negative bacteria. They are one of the most frequent causes of intramammary infections3; mainly causing clinical mastitis.
Clinical mastitis caused by Klebsiella species are characterized by consistently high somatic cell counts, higher rates of culling and voluntary quarter dry off4 compared to clinical cases caused by Escherichia coli—another Gram-negative bacterium.
The most isolated Klebsiella species from cases of clinical mastitis are: Klebsiella pneumoniae and Klebsiella oxytoca. Both species are frequently found in the farm environment, body sites of cow, and swabs taken from teat-end5,6,7.
Why is Klebsiella, a mastitis-causing pathogen, important to human health?
In humans, Klebsiella causes one-third of opportunistic Gram-negative infections, including wound, urinary tract, and hospital-acquired (i.e., newly acquired infections that are contrated within a hospital environment) infections8. Klebsiella pneumoniae is commonly associated with community-acquired infections9 such as liver abscess, meningitis, or endophthalmitis10.
Because this pathogen is very prevalent among humans, it is possible that human Klebsiella could acquired AMR genes from the environment11-12, which might make this bacterium not susceptible to the current antimicrobials available for treating those infections.
Due to the usage of antimicrobial for treating dairy cows, it has been hypothesized that bacteria at the farm setting can acquired AMR and pose a great risk to people2. However, there is no conclusive data to prove that hypothesis. So far, we can say that there is no increase of AMR in mastitis-causing pathogens on dairy farms13-16. Surveillance is a key factor to determine whether AMR is increasing in mastitis-causing pathogens.
Antimicrobial resistance of Klebsiella species
Recently, we summarized AMR trends in Klebsiella isolates cultured from milk samples submitted to the Wisconsin Veterinary Diagnostic Laboratory (WVDL) for bovine mastitis testing between 2008 and 2019. They tested 483 Klebsiella isolates cultured from 63,841 milk samples and ten antimicrobials were tested: ampicillin, penicillin, erythromycin, oxacillin + 2% NaCl, pirlimycin, penicillin/novobiocin, tetracycline, cephalothin, ceftiofur, and sulphadimethoxine. Most of these antimicrobial compounds are currently used in products intended for preventive and therapeutic care of dairy cattle.
Klebsiella have innate mechanisms to resist the antimicrobial effect of ampicillin, penicillin, erythromycin, oxacillin + 2% NaCl, pirlimycin, penicillin/novobiocin action, thus, AMR was more than 90% for all these antimicrobials for 2008-2019.
We found that ceftiofur AMR varied through 2008 and 2019 and increased between 2016 and 2017 (Figure 2). We could not explain why AMR increased but we hypothesized that it might be associated with ceftiofur formulations being commonly used for treatment or prevention of intramammary infections in dairy cows.
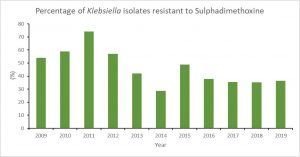
For sulphadimethoxine, we found that resistance decreased through 2008 and 2019 (Figure 3). This result might reflect that farmers follow label specification and use sulphadimethoxine only for dairy calves, heifers, and beef cattle.
Except for ceftiofur, we did not find increasing AMR trends among Klebsiella isolates cultured from milk samples submitted to the WVDL for bovine mastitis testing.
Conclusion
Antimicrobial resistance is an important issue on dairy operations. People in our communities expect farmers to decrease use of antimicrobial on dairy farms. Although, there is not conclusive data about the relationships between AMR development in animal pathogens, the farm environment, and human health, we need to provide adequate safety measures to reduce the likelhood of AMR. Particularly, dairies could introduce procedures for staff who work closely with cattle—dairy workers—to decrease any chance of transmission of animal pathogens carrying AMR genes to those workers.
Take Home Messages
- Antimicrobials should be used in animals who will benefit from it—to determine which animals should receive antimicrobials consult with your herd veterinarian.
- Consult with you herd veterinarian to develop a mastitis treatment protocol using a system that allows you to know the mastitis-causing pathogen.
- Remember antimicrobial (based on label specifications) kill or reduce growth of bacteria—they do not increase milk production or decrease somatic cell count.
- Stay tuned and check educational resources in the UW Division of Extension website for more information about safety practices, personal protective equipment, and different management practices that could help to reduce risk of AMR.
References
[1] FDA (Food and Drug Administration). 2018. Extralabel use and antimicrobials. Accessed March 8, 2019. https://www.fda.gov/animal-veterinary/antimicrobial-resistance/extralabel-use-and-antimicrobials.
[2] Sifferlin, A. 2017. Here’s how to cut antibiotic use in animals. TIME Health. Accessed April 20, 2020. https://time.com/4961051/antibiotics-animals/
[3] Oliveira, L., C. Hulland, and P. L. Ruegg. 2013. Characterization of clinical mastitis occurring in cows on 50 large dairy herds in Wisconsin. J. Dairy Sci. 96:7538–7549.
[4] Fuenzalida, M. J. and P. L. Ruegg. 2019. Negatively controlled, randomized clinical trial to evaluate intramammary treatment of nonsevere, gram-negative clinical mastitis. J. Dairy Sci. 102:5438–5457.
[5] Munoz, M. A., F. L. Welcome, Y. H. Schukken, and R. N. Zadoks. 2008a. Molecular epidemiology of two Klebsiella pneumoniae mastitis outbreaks on a dairy farm in New York State. J. Clin. Microbiol.45:3964–3971.
[6] Zadoks, R. N., H. M. Griffiths, M. A. Munoz, C. Ahlstrom, G. J. Bennett, E. Thomas, and Y. H. Schukken. 2011. Sources of Klebsiella and Raoultella species on dairy farms: be careful where you walk. J. Dairy Sci. 94:1045–1051.
[7] Rowbotham, R. F., and P. L. Ruegg. 2016. Bacterial counts on teat skin and in new sand, recycled sand, and recycled manure solids used as bedding in freestalls. J. Dairy Sci. 99:6594–6608.
[8] Navon-Venezia, S. K. Kondratyeva, and A. Carattoli. 2017. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Reviews. 41:252–275.
[9] Paterson, D. L., K. L. K. Siu, and F. Chang. 2002. Klebsiella species (K. pneumoniae, K. oxytoca, K. ozaenae and K. rhinoscleromatis) in Antimicrobial therapy and vaccines: Microbes. Vol. I. 2nd ed., V. L. Yu, R. Weber, and D. Raoult (Eds.), Apple Trees Production, LLC.
[10] Ko W., D. L. Paterson, A. J. Sagnimeni, D. S. Hansen, A. Von Gottberg, S. Mohapatra, J. M. Casellas, H. Goossens, L. Mulazimoglu, G. Trenholme, K. P. Klugman, J. G. McCormack, and V. L. Yu. 2002. Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg. Infect. Dis. 8:160–166.
[11] Wyres, K. L., and K. E. Holt. 2018. Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Curr. Opin. Microbiol. 45:131–139.
[12] Nóbrega, D. B., M. Guiduce, F. Guimaraes, D. Riboli, M. Cunha, H. Langoni, J. Pantoja, and S. Lucheis. 2013. Molecular epidemiology and extended-spectrum beta-lactamases production of Klebsiella pneumoniae isolated from three dairy herds. Pesq.Vet. Bras. 33:855–859.
[13] Fuenzalida, M. J., E. M. Furmaga, and N. Aulik. 2020. Short Communication: Antimicrobial Resistance in Klebsiella Species from Milk Specimens Submitted for Bovine Mastitis Testing at the Wisconsin Veterinary Diagnostic Laboratory, 2008-2019. Submitted to Journal of Dairy Science.
[14] Makovec, J. A., and P. L. Ruegg. 2003. Antimicrobial resistance of bacteria isolated from dairy cow milk samples submitted for bacterial culture: 8,905 samples (1994-2001). J. Am. Vet. Med. Assoc. 222:1582–1589.
[15] Nóbrega, D. B., J. De Buck, and H. W. Barkema. 2018. Antimicrobial resistance in non-aureus staphylococci isolated from milk is associated with systemic but not intramammary administration of antimicrobials in dairy cattle. J. Dairy Sci. 101:7425–7436.
[16] Erskine, R. J., R. D. Walker, C. A. Bolin, P. C. Bartlett, and D. G. White. 2002. Trends in antibacterial susceptibility of mastitis pathogens during a seven-year period. J. Dairy Sci. 85:1111–1118.